Foundation News Giving back in gratitude For people who have lost a loved one to ALS, the holidays are a time of reflection and gratitude to those who cared for family and friends during their ALS journey. Victor was 14 …
November 2022 Foundation eNews
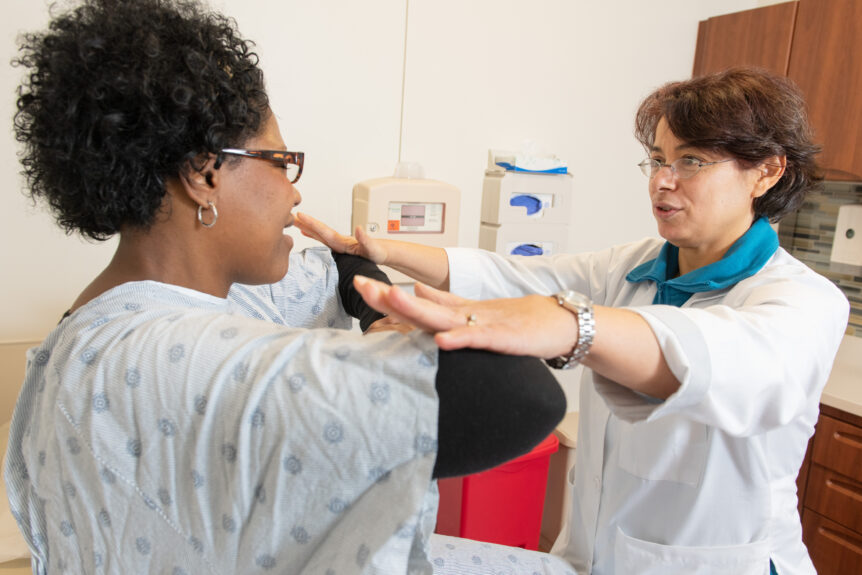
Foundation News Grants that make a difference The Les Turner ALS Foundation offers several grant programs to help provide financial assistance, independence and empowerment for people living with ALS. In 2022, we will have funded nearly 80 grants – made possible …
October 2022 Foundation eNews
Foundation News Register for NINDS ALS Strategic Planning Workshop Tomorrow, the draft of the National Institute of Neurological Disorders and Stroke (NINDS) ALS Strategic Plan, is being made available to the public. The Les Turner ALS Foundation has been an …
New results show promise in treatment of SOD1-ALS
New results from the Phase 3 clinical trial of tofersen, a drug in development by Biogen, have shown promise in treatment of superoxide dismutase 1 (SOD1) amyotrophic lateral sclerosis (ALS). According to the 12-month data, earlier initiation of treatment with …
NU-9 Update: Experimental drug repairs upper motor neurons in mouse model
As connecting fibers between motor neurons, axons help deliver vital messages between the brain and the spinal cord. For people living with ALS, deteriorating axons cause that connection to break, contributing to paralysis and death. Early research on NU-9, an …
Breaking News: FDA Approves Oral Edaravone
This story has been updated with a link to the informational webinar about the new medication. The U.S. Food and Drug Administration has approved RADICAVA ORS®, an oral form of edaravone that reports the same efficacy as RADICAVA® for intravenous infusion. …
Research News from the Les Turner ALS Center at Northwestern Medicine
The 40+ research scientists at the Les Turner ALS Center at Northwestern Medicine investigate a wide range of biologic mechanisms in ALS basic research. We are pleased to announce the recent publication of three new research papers from some of …
Our Oral Testimony to the FDA Advisory Committee on the Approval of AMX0035
Open Public Hearing FDA Advisory Committee on AMX0035 March 30, 2022 Good afternoon. My name is Andrea Pauls Backman, CEO of the Les Turner ALS Foundation. My only disclosure is that the Les Turner ALS Foundation receives less than 2% …
Comments on Amylyx Pharmaceuticals’ New Drug Application for AMX0035
RE: Docket No. FDA-2018-N-0410 Dear FDA Advisory Committee: Founded in 1977, the Les Turner ALS Foundation is the oldest independent ALS group in the country. For 45 years, it has been our mission to provide the most comprehensive care and …
Response to NINDS Request for Information on ALS Strategic Plan
February 11, 2022 Dear NINDS, As we set our sights on shaping the strategic future of ALS research, we thank you for the opportunity to identify key research and priorities at NIH and beyond. We also recognize …