RE: FDA-2022-N-0691 Dear FDA Advisory Committee: We write to express our strong support for the approval of tofersen under the FDA’s Accelerated Approval Program. Our organization’s long history of scientific research into SOD1-ALS and our depth of experience supporting people …
Calming the destructive cells of ALS by two independent approaches
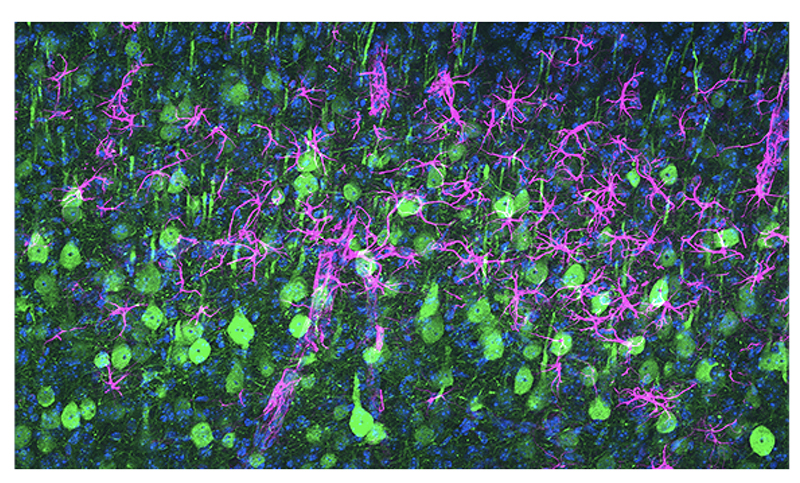
Scientists from the Les Turner ALS Center at Northwestern Medicine have discovered two ways to preserve diseased upper motor neurons that would normally be destroyed in ALS, based on a study in mice. Upper motor neurons initiate movement, and they …
2022 Impact Report
Your support of the Les Turner ALS Foundation is felt by people living with ALS, caregivers, families and loved ones everywhere. By making a donation or volunteering your time, you helped ensure they had the support they needed – and …
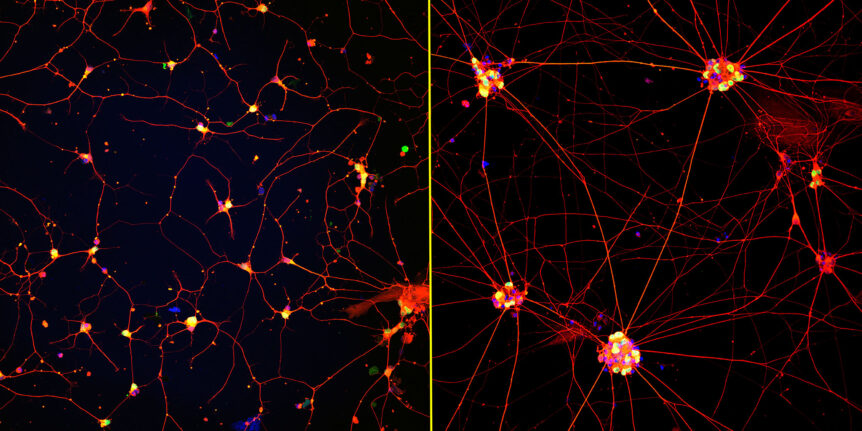
Mature ‘lab grown’ neurons hold promise for ALS and other neurodegenerative diseases
Researchers at Northwestern University and the Les Turner ALS Center at Northwestern Medicine have created the first highly mature neurons from human induced pluripotent stem cells (iPSCs), a feat that opens new opportunities for medical research and potential transplantation therapies …
FDA Awards 19 Grants and Two Contracts Related to Rare Diseases, including ALS
On Monday October 17, 2022, the U.S. Food and Drug Administration announced it has awarded 19 new grants and two new contracts totaling more than $38 million in funding over the next four years to support clinical trials, natural history …
Newly published data on tofersen shows success in treating SOD1-ALS
New results published this week provide further evidence that tofersen, a drug in development by Biogen, may be effective in slowing the progression of SOD1-ALS. As reported in the New England Journal of Medicine, the Phase 3 clinical trial (VALOR) …
FDA grants priority review for new SOD1-ALS treatment
The U.S. Food and Drug Administration (FDA) has accepted a New Drug Application for tofersen, a drug in development by Biogen for treatment of superoxide dismutase 1 (SOD1) amyotrophic lateral sclerosis (ALS) – and granted priority review for the application. …
New results show promise in treatment of SOD1-ALS
New results from the Phase 3 clinical trial of tofersen, a drug in development by Biogen, have shown promise in treatment of superoxide dismutase 1 (SOD1) amyotrophic lateral sclerosis (ALS). According to the 12-month data, earlier initiation of treatment with …
NU-9 Update: Experimental drug repairs upper motor neurons in mouse model
As connecting fibers between motor neurons, axons help deliver vital messages between the brain and the spinal cord. For people living with ALS, deteriorating axons cause that connection to break, contributing to paralysis and death. Early research on NU-9, an …
Research News from the Les Turner ALS Center at Northwestern Medicine
The 40+ research scientists at the Les Turner ALS Center at Northwestern Medicine investigate a wide range of biologic mechanisms in ALS basic research. We are pleased to announce the recent publication of three new research papers from some of …