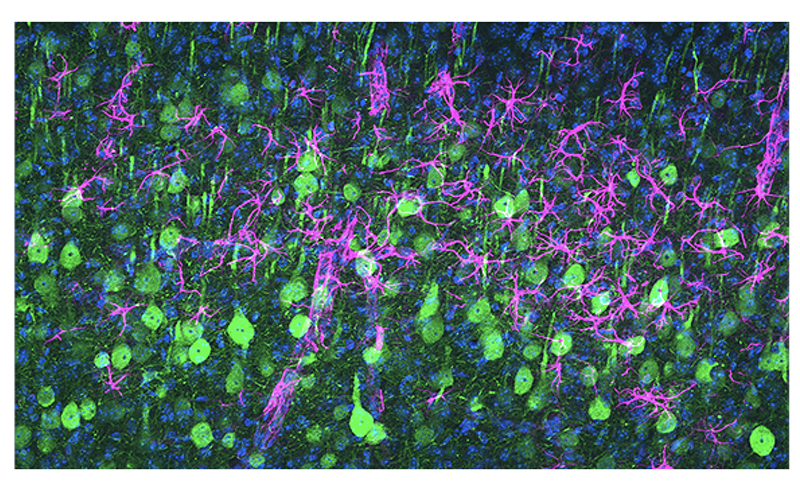
As many as 2% of all ALS cases have been linked to mutations in the gene NEK1, making it one of the top-known causes of the disease. But it wasn’t known how the mutated gene disrupts the function of the …
Nearly $1 Million in ALS Grants Awarded for 2023
In 2023, the Les Turner ALS Foundation is funding nearly $1 million in ALS research grants and clinic and endowment support at the Les Turner ALS Center at Northwestern Medicine. This is the second year in a row that funding …
Lois Insolia ALS Clinic Opens Enrollment for New HEALEY ALS Platform Trial Regimen
The Lois Insolia ALS Clinic at the Les Turner ALS Center at Northwestern Medicine is now an active site for Regimen F of the HEALEY ALS Platform Trial. The focus of Regimen F is ABBV-CLS-7262, an investigational product being developed …
Comments to the FDA Advisory Committee in Support of Tofersen Approval
RE: FDA-2022-N-0691 Dear FDA Advisory Committee: We write to express our strong support for the approval of tofersen under the FDA’s Accelerated Approval Program. Our organization’s long history of scientific research into SOD1-ALS and our depth of experience supporting people …
Calming the destructive cells of ALS by two independent approaches
Scientists from the Les Turner ALS Center at Northwestern Medicine have discovered two ways to preserve diseased upper motor neurons that would normally be destroyed in ALS, based on a study in mice. Upper motor neurons initiate movement, and they …
2022 Impact Report
Your support of the Les Turner ALS Foundation is felt by people living with ALS, caregivers, families and loved ones everywhere. By making a donation or volunteering your time, you helped ensure they had the support they needed – and …
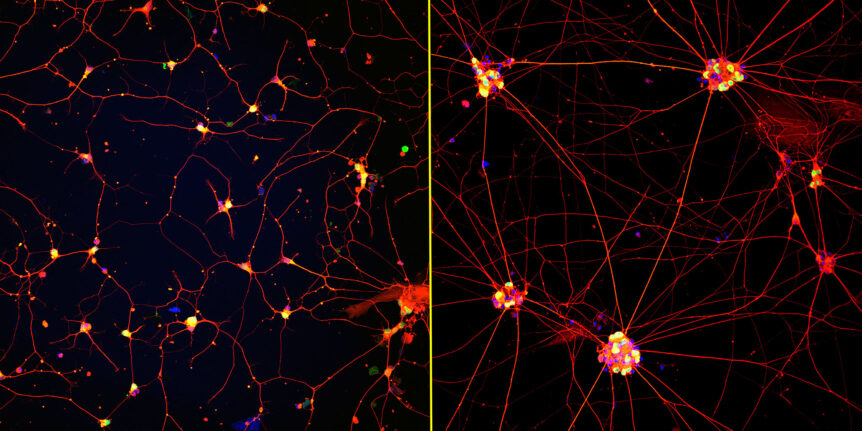
Mature ‘lab grown’ neurons hold promise for ALS and other neurodegenerative diseases
Researchers at Northwestern University and the Les Turner ALS Center at Northwestern Medicine have created the first highly mature neurons from human induced pluripotent stem cells (iPSCs), a feat that opens new opportunities for medical research and potential transplantation therapies …
FDA Awards 19 Grants and Two Contracts Related to Rare Diseases, including ALS
On Monday October 17, 2022, the U.S. Food and Drug Administration announced it has awarded 19 new grants and two new contracts totaling more than $38 million in funding over the next four years to support clinical trials, natural history …
Newly published data on tofersen shows success in treating SOD1-ALS
New results published this week provide further evidence that tofersen, a drug in development by Biogen, may be effective in slowing the progression of SOD1-ALS. As reported in the New England Journal of Medicine, the Phase 3 clinical trial (VALOR) …
FDA grants priority review for new SOD1-ALS treatment
The U.S. Food and Drug Administration (FDA) has accepted a New Drug Application for tofersen, a drug in development by Biogen for treatment of superoxide dismutase 1 (SOD1) amyotrophic lateral sclerosis (ALS) – and granted priority review for the application. …